The Invisible Patient: Women’s Health in Medicine & Research
Zsofia Hesketh is an interdisciplinary scholar and public health professional with a background in philosophy, medical research, and clinical medicine. She is completing her MSc Translational Medicine as a visiting student at Sorbonne Médecine, Paris, where she is focusing on gynaecology and neglected aspects of women’s health.
This article is part of the intersections theme.
edited by Kenia & heini, reviewed by laura suominen, illustrated by kenia & sophie hoetzel, read by emmi olkkonen.
“The prevention, management and therapeutic treatment of many common diseases does not reflect the most obvious and most important risk factors for the patient: sex and gender”
Prof. Vera Regitz-Zagrosek, founder of the Gender in Medicine (GiM) Institute at Charite, Berlin
Welcome to our new mini-series on sex-sensitive (also called “sex-stratified”) medicine, the practice of ensuring that patients’ unique needs are met during prevention, diagnosis and treatment by considering how their sex and gender characteristics influence their health. Throughout the next months, we will explore the intersections between sex, gender, public health and medicine and dive deep into three areas of healthcare where important sex and gender differences have been found or remain to be uncovered – with hormones representing a linking thread between all forthcoming topics.
This first instalment discusses the overall theme of sex-sensitive medicine by explaining why it is so important and enumerating several core examples of how sex and gender are implicated in health.
The need for sex-sensitive medicine
Sex-sensitive medicine is a fundamentally important yet understudied aspect of modern medical care, which often falls often short particularly when it comes to women and girls. Neglect of the dimensions of sex and gender has manifested most acutely in medicine as a relative ignorance and low prioritisation of women’s health, meaning that, for example, conditions which occur more frequently or exclusively in women, like migraine and endometrosis, have remained undertreated and underdiagnosed.
Studies have also shown that, on average, medical professionals take female patients’ pain levels less seriously because pain is accepted as a ‘normal’ part of living as a woman. Pain scale questionnaires (see Figure 1), in use for only several decades, have been an important development in this regard. They provide a quantification measure from 1-10 to evaluate and understand what patients, like women and girls suffering from (pre-)menstrual pain, is feeling more objectively.
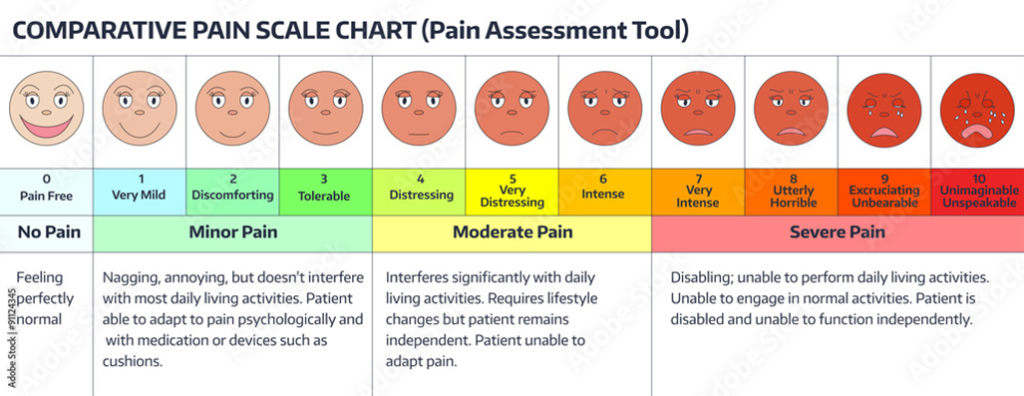
Figure 1. Example of a pain scale chart, used to help patients describe the intensity of their symptoms and the level of interference with their daily activities. Source: Stat Magazine.
But the issue of gender equity in healthcare runs much deeper than you might think. Significant gaps exist in our understanding of female-specific physiology and molecular processes, both in healthy and diseased states. This translates into incomplete knowledge of the specifics of disease risk, cause, and progression, and little attention paid to developing evidence-based treatments that are well-adapted to the female body.
Neglect of sex and gender in research
The terms sex and gender, often used interchangeably in everyday speech, carry specific nuances in science and medicine that are important to understand before we start diving deep into sex-stratified medicine.
“Sex” refers to the biological, chromosomal (XX or XY) categorisation of an individual, which in turn influences the secondary sex characteristics they develop such as testicle versus breast growth, or the presence of facial hair or lack thereof. Meanwhile, “gender” can be understood as the collection of behaviours and roles commonly associated with each sex that emanate partially from sex-induced tendencies and partially from societal expectations and conditioning. This includes temperament, the kind of clothing worn, the length of one’s hair, or the type of job commonly selected. The two concepts are therefore distinct yet strongly intertwined as they can reciprocally influence each other. For example, testosterone promotes aggression and risk-taking, which is also societally expected in men, reinforcing the exhibition of these traits beyond the biological threshold.
Gender-blindness, identified as an obstacle to gender equity in health care, is the “nonawareness of the fact that a great deal of knowledge is based on research performed in men”, which translates to the default patient implicitly being assumed to be male. If we retrace the steps between treatment administration and drug development and testing, women are in fact underrepresented in both clinical and pre-clinical trials (e.g. female models, like mice, rats or larger mammals). Certain cardiovascular drug trials show an especially significant disparity, with a UK study finding that only 16% of women are included in statin trials compared to the 45% who use statins in the population. A more general recent analysis of 1,433 clinical trials including 302,664 participants found that, on average, only 41.2% were female.
Another important shortcoming is the lack of studies analysing the differences in medical treatments and therapies between women and men. Because sex and gender affect a wide range of physiological mechanisms, they also impact what goes both right and wrong within them. Lower body surface in women, differences in kidney function, drug resorption and metabolism by liver enzymes and excretion cause significant differences in pharmacokinetics (how the body processes substances introduced into it, like medications and nutrients). Many drugs, therefore, require different dosing to achieve optimal effects.
In addition, differences in pharmacodynamics (the effect of said substances on the organism they are introduced into) are also widespread, causing sex-specific effects. Many sex and gender differences have been reported, for example, in the efficiency and adverse effects of heart failure drugs (such as digitalis, angiotensin-converting enzyme inhibitors and anti-arrhythmics), but also in painkillers, neuropsychiatric, anticancer and cardiovascular drugs.
Newly developed medications, modified drug dosage, combination and contraindication regimens, or nutritional and exercise plans specifically designed for optimal impact in women are some unexplored interventions that account for sex and gender and are likely to be more effective and trigger fewer side effects. This could lead to better health outcomes and a higher likelihood of sticking to the treatment than usual ‘one-size-fits-all’ approaches.
Beyond pregnancy: female patients as complete individuals
There is one medical context in which particular attention is already somewhat paid to the specifics of female physiology. This is obstetrics and gynaecology, a field that intimately links women’s health with the health of their offspring and their potential to gestate a child.
An important and unique aspect of female physiology, our medical knowledge on this field might be ahead of others, but still demands more attention. The jury is very much still out, for example, on which foods are safe or unsafe to consume during pregnancy; the type of exercises best suited for expectant mothers; the complications that can occur during each trimester and, of course, the often crude and uncomfortable way in which labour and birth are dealt with in the hospital setting. This goes from uncomfortable delivery positions to traumatic procedures and potential major surgery, all with blurred lines on patient understanding and consent.
Nonetheless, the health of women as a group with particular shared characteristics – and, conversely, women as individuals in their own right and not as potential mothers – is rarely addressed across other gynaecological research questions and medical specialities. The broader implications of female-specific organs and apparatus are notoriously disregarded or forgotten. Coming to mind are once again the debilitating symptoms of endometriosis, the relation between the menstrual cycle and proneness to neurological alterations like epilepsy and migraine, and the features of menopause.
On a frustrating note that illustrates this lack of representation for women patients beyond pregnancy, when searching for suitable images for the article, every royalty-free site on which I searched “female patient” came up with hundreds of images of various pregnant women. Why can’t we just have a woman sitting in a doctor’s office?
The importance of female pleasure for good relational and psychological health has also received little attention: despite centuries of anatomical dissection and drawings, the first detailed anatomical description of the internal clitoris was only published in 1998, saying little about its functions.
Knowledge gaps surrounding the female body
There are clear biological differences that arise from our DNA and which affect many bodily systems. Most obvious is the influence of steroid sex hormones, molecules that, among other functions, are responsible for developing sex-specific characteristics and thus “represent what makes females different from males” biologically.
Contrary to popular belief, the three main types of sex-specific hormone, oestrogens, progesterone and testosterone, are naturally present in both females and males. What is different between sexes are their production sites (testes vs. ovaries and pituitary gland), their blood concentrations, and their interactions with different organs, including more constant levels present in males versus an oscillating pattern characteristic of females following the menstrual cycle. These differences then impact body composition (e.g. fat-to-muscle ratio and bone mass), nutritional needs, circadian rhythms, temperament and much more.
On the micro-scale, findings point to still more intricate differences. This is the case with ion channels, gatekeeper proteins on the surface of our cells allowing ions necessary for life like sodium, potassium and calcium to move in and out. Ion channels can be set to open in both directions, only one direction, or to stay closed. Interestingly, the type and quantity of ion channels on the surface of certain cells in women, such as blood vessel membranes, have been found to be different than in men.
As ion channels govern the substances that enter and exit our cells – from ingested food compounds to pharmaceutical molecules – such differences can potentially have a very real impact on the functioning, maintenance, degradation and regeneration of female cells and tissues. These changes, possibly derived from specific and complex hormonal interactions over the life course, can then lead to increased proneness to conditions such as arrhythmia and heart failure. For example, certain ions entering the vagal nerves or heart muscle cells can increase or decrease heart rate or change its regularity, influencing how fast or strongly it beats.
In another example of cellular-scale sex differences, let’s talk about cancer. Sex-specific genes and hormones can impact the likelihood of developing certain types of cancer, including some unrelated to reproductive functions. To give you an idea, consider the following example. One of the mechanisms that fuel cancer development is the accumulation of defects in DNA damage response pathways, a complex set of molecular interactions that sense DNA lesions. Think about them as warning systems: depending on the lesions’ severity, these pathways will halt DNA replication in the cell, trigger its repair or destroy defective cell (apoptosis), thereby preventing the uncontrolled cell division that leads to cancer.
“TSPYL2” and “PARP1” are two proteins that are involved in damage response DNA pathways, helping to halt cancer cell replication. They have been found to accumulate in stressed and cancer-susceptible cells, but they do so at different rates and timings in males and females. These differences are thought to occur due to the presence or absence of the Y chromosome, which exists only in males and contains genes that modulate (meaning to either increase or decrease) the activity of these proteins.
One of these modulating genes is called “Sex-determining region R” or “SRY” for short. While crucial for the foetus, as it is responsible for the development of male sex characteristics such as the testes that secrete testosterone, its expression in adult males has been associated with reduced activity of the TSPYL2 protein to respond to DNA damage in liver cells. This makes liver screening in men particularly important, and allows for an evidence-based redirection of resources to other types of cancer in women. Conversely, SRY indirectly improves the activity of PARP1 in certain tissues, like male breast tissue, as increased testosterone secretion may promote better DNA damage response by this protein. On the other hand, since oestrogen is responsible for breast tissue growth, it is more often implicated in excessive cell division and thus breast cancer in females.
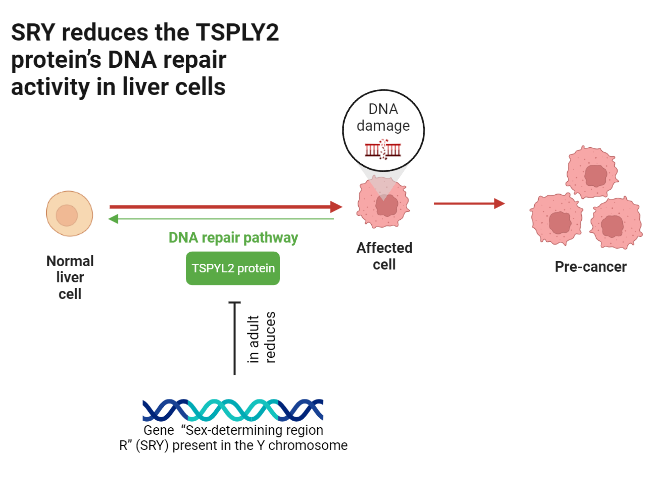
This is an example of how emerging evidence of sex-specific differences, in individual genes as well as hormones indirectly governed by them, can either promote or reduce cancer suppression and thus create new opportunities for precision medicine.
Coming next: exploring menstrual and reproductive health
As described in this article, the need to integrate a sex and gender perspective into medical research, clinical work and public health is more apparent than ever. The purposeful and rigorous pursuit of understanding female physiology could affect the quality of care of a myriad diseases, and prevent or delay the progression of many more.
Next month’s article will revolve around menstrual and reproductive health, which have seen several important – though still insufficient – leaps of progress in past decades. Until recently, the localised and systemic side effects of contraceptive hormone supplementation have been little questioned, with routine and indiscriminate prescription of synthetic hormones even to girls on the lower end of their teenage years. On the other hand, the deleterious effects of hormone supplementation must be considered next to the greater reproductive control for women they represent.
Follow The Science Basement on Twitter or Instagram and join me next month to explore this and other reproductive topics, such as little-known aspects of the menstrual disturbances and menopause.
references
Bierer, Barbara E., et al. “Advancing the Inclusion of Underrepresented Women in Clinical Research.” Cell Reports Medicine, vol. 3, no. 4, Apr. 2022, p. 100553, doi:https://doi.org/10.1016/j.xcrm.2022.100553.
Cardano, Miriana, et al. “Sex Disparities in DNA Damage Response Pathways: Novel Determinants in Cancer Formation and Therapy.” IScience, vol. 25, no. 3, 18 Mar. 2022, p. 103875, pubmed.ncbi.nlm.nih.gov/35243237/, https://doi.org/10.1016/j.isci.2022.103875. Accessed 9 Nov. 2022.
Cardano, Miriana, et al. “Sex Specific Regulation of TSPY-like 2 in the DNA Damage Response of Cancer Cells.” Cell Death & Disease, vol. 14, no. 3, 15 Mar. 2023, pp. 1–12, www.nature.com/articles/s41419-023-05722-2, https://doi.org/10.1038/s41419-023-05722-2. Accessed 3 Apr. 2023.
Costa, Sarah, et al. “The Link between Sex Hormones and Susceptibility to Cardiac Arrhythmias: From Molecular Basis to Clinical Implications.” Frontiers in Cardiovascular Medicine, vol. 8, 17 Feb. 2021, https://doi.org/10.3389/fcvm.2021.644279. Accessed 5 Mar. 2023.
Hudson, Nicky. “The Missed Disease? Endometriosis as an Example of “Undone Science.”” Reproductive Biomedicine & Society Online, vol. 14, Mar. 2022, pp. 20–27, www.ncbi.nlm.nih.gov/pmc/articles/PMC8517707/, https://doi.org/10.1016/j.rbms.2021.07.003.
Lagro-Janssen T. The Palgrave Handbook of Gender and Healthcare. Berlin, Germany: Springer; 2010. Sex, gender and health: developments in medical research; pp. 405–420.
Lauretta, R., et al. “Gender in Endocrine Diseases: Role of Sex Gonadal Hormones.” International Journal of Endocrinology, vol. 2018, 21 Oct. 2018, pp. 1–11, https://doi.org/10.1155/2018/4847376. Accessed 2 July 2020.
Miguel-Aliaga, Irene. “Let’s Talk about (Biological) Sex.” Nature Reviews Molecular Cell Biology, 23 Feb. 2022, https://doi.org/10.1038/s41580-022-00467-w. Accessed 7 Mar. 2022.
O’Connell, H. E., Sanjeevan, K. V., & Hutson, J. M. (2005). Anatomy of the clitoris. Journal of Urology, 174(4 Pt 1), 1189–1195. https://doi.org/10.1097/01.ju.0000173639.38898.cd.
Regitz-Zagrosek, Vera. “Sex and Gender Differences in Health.” EMBO Reports, vol. 13, no. 7, 15 June 2012, pp. 596–603, www.ncbi.nlm.nih.gov/pmc/articles/PMC3388783/, https://doi.org/10.1038/embor.2012.87.
Samulowitz, Anke, et al. ““Brave Men” and “Emotional Women”: A Theory-Guided Literature Review on Gender Bias in Health Care and Gendered Norms towards Patients with Chronic Pain.” Pain Research and Management, vol. 2018, no. 1, 25 Feb. 2018, pp. 1–14, https://doi.org/10.1155/2018/6358624.
Sosinsky, Alexandra Z., et al. “Enrollment of Female Participants in United States Drug and Device Phase 1–3 Clinical Trials between 2016 and 2019.” Contemporary Clinical Trials, vol. 115, Apr. 2022, p. 106718, https://doi.org/10.1016/j.cct.2022.106718.
Westergaard, David, et al. “Population-Wide Analysis of Differences in Disease Progression Patterns in Men and Women.” Nature Communications, vol. 10, no. 1, 8 Feb. 2019, pp. 1–14, www.nature.com/articles/s41467-019-08475-9, https://doi.org/10.1038/s41467-019-08475-9.