Can we outsmart superbugs? The latest breakthroughs in the field of antimicrobial resistance
Maitri Mishra is a PhD student in Biotechnology. Her research topic is broadly on finding innovative nanotechnology-based solutions to drug-resistant infections. She likes to think of herself as a materials scientist obsessed with solving math problems in her free time. she also likes to code, bake, strum, and sleep!
This article is part of the eureka moments and turning points theme.
edited by mimmu and Saara and reviewed by Marko virta. illustrated by kenia, sophie, and vicky. Should you have any comments, please let us know!
During the pre-antibiotic era there was no cure for bacterial infections, and thus contracting one was considered a death sentence. The discovery of antibiotics in 1928 by Alexander Fleming was like a bright light emerging from the darkness, a beacon of light among all the despair arising from epidemics of deadly infections. The discovery of antibiotics tremendously improved medical care all over the world. Antibiotics have become a cornerstone of healthcare and we use them to treat life-threatening illnesses and aid in complex surgeries, such as organ transplantation.
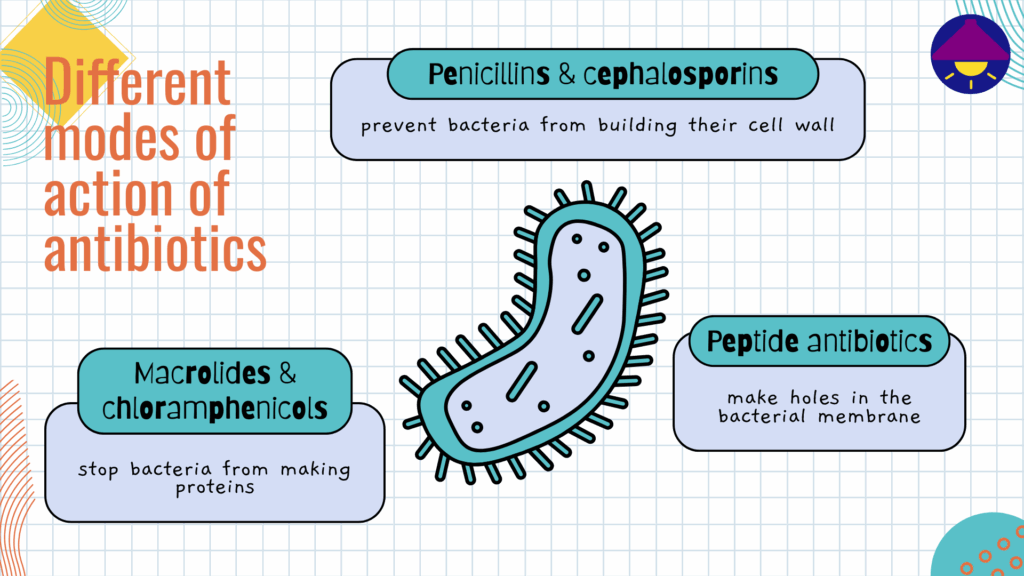
Antibiotics can be broad- or narrow-spectrum depending on how many bacterial strains they affect. Various natural, synthetic, and semi-synthetic antibiotics are currently in use for treating infections, and they are divided into different classes (such as penicillins, cephalosporins, macrolides, peptides, etc.) based on the similarities and differences in their structures and modes of action (see details in the image below). Unfortunately, the gradual overuse and widespread misuse of these miracle drugs has led to the rise of a phenomenon called antimicrobial resistance, or AMR for short. This type of resistance occurs when microorganisms like bacteria, fungi, parasites, and viruses evolve in a way that makes the antimicrobial drugs unable to kill them.
Although antimicrobial resistance has been documented since the 1940s, the introduction of antibiotics into animal feed gave AMR a boost, leading to the emergence of methicillin resistance in 1960. The rising frequency of this phenomenon means that infections are getting increasingly difficult to treat, leading to longer illnesses, increased healthcare costs, and eventually an increased risk of mortality. Microbes, especially bacteria, are gradually becoming unaffected by the presence of even multiple antibiotics, which has given rise to multidrug-resistant (MDR) bacteria. Antimicrobial resistance threatens to send the world back to a pre-antibiotic era when common infections could also turn deadly.
The World Health Organization (WHO) considers antimicrobial resistance one of the top ten threats to human health and is taking steps to combat it globally. Researchers are now working towards developing rapid diagnostic methods for its detection, understanding its molecular processes, and designing strategies against resistant infections.
Because there is no single magic wand we can wave to eradicate antimicrobial resistance, a combination of all these efforts is needed to direct the world towards a path where management and mitigation of the spread of infections caused by MDR bacteria are possible. In this article I’ll introduce you to some of the most promising solutions and significant research on the subject in 2024.
So, let’s dive in!
New antibiotics in the era of resistance
Urinary tract infections (UTIs) are one of the most common infections worldwide – one in two women suffer from them in their lifetime! They occur when bacteria enter the urinary tract and begin to spread in the bladder, and are known to affect women more often than men. Antimicrobial resistance in bacteria causing UTIs is currently on the rise, but, luckily, we’ve also made breakthroughs in novel antibiotics for treatment of urinary tract infections.
In 2024, the Center for Drug Evaluation and Research at the Food and Drug Administration (FDA) gave the green light to four antibiotics, three of which were approved for treatment of urinary tract infections. In February 2024, FDA approved Exblifep for treating complicated infections. Exblifep acts against cephalosporin-resistant bacteria, such as E. coli and pneumonia, which produce enzymes that make them resilient to antibiotics. Imagine that the antibiotic is trying to disable the infection’s weaponry!
Next, Pivya was approved in April 2024 for UTIs in women as the first oral antibiotic in two decades. A third antibiotic for treating UTIs, Orlynvah, was approved in October 2024. As opposed to Pivya, Orlynvah is meant for women who have limited oral antibiotic treatment options, and for treating infections caused by specific bacteria, such as E. coli.
The treatment of urinary tract infections is at the centre of focus here because both the infection and antibiotic resistance of UTI-causing bacteria are so common, but we’ve seen progress in fighting other infections alike. For example, FDA also approved a new broad-spectrum cephalosporin antibiotic, Zevtera, in April 2024. It’s a treatment against community-acquired bacterial pneumonia (which I will simply refer to as pneumonia in this article), bloodstream infections caused by the Staphylococcus bacteria, and acute bacterial skin and skin structure infections. However, in November 2024, generic formulations of Zevtera were discontinued in the USA and some other countries. The exact reasons for this discontinuation haven’t been publicly disclosed, but the widespread speculation is that Zevtera was discontinued due to difficulties in a sustained supply of the product.
On the horizon: Antibiotics under development
While some new antibiotics were approved, the hunt for even more antibiotics also picked up steam in 2024 worldwide. For example, Nafithromycin is a macrolide antibiotic indigenously developed in India, made to target pneumonia. What makes Nafithromycin exciting is that it is 10 times more effective than current treatment strategies and has a shorter treatment duration.
On the other side of the globe, researchers in Germany have created Darobactin D22, an improvement on the naturally produced heptapeptide antibiotic, darobactin. Ongoing research shows promising results against bacteria, like E. coli, that are known to develop antibiotic resistance easily. Similarly, researchers at Harvard University and the University of Illinois at Chicago developed a synthetic compound called Cresomycin, that has been proven effective against some strains of multidrug-resistant bacteria.
Phage therapies and research take us beyond antibiotics
Did you know that antibiotics are not the only way to combat antimicrobial resistance? While antibiotics are important tools against resistant bacteria, they are just a part of a larger scheme of eradicating antimicrobial resistance. Firstly, WHO recognizes vaccines as crucial tools to overcome the spread of infectious pathogens, and thus the need for consuming antibiotics. The year 2024 saw some vaccines in different phases of development against diseases such as tuberculosis and shigellosis.
Secondly, in 2024, researchers used phage therapy to treat patients with chronic lung infections caused by antibiotic-resistant bacteria. Phage therapy is a treatment form that gets its name from “bacteriophages” – viruses that kill bacteria – which are then used to treat bacterial infections. And its usefulness doesn’t stop at lung infections: in 2024 phage therapies were also used to treat wound infections, including those caused by the methicillin-resistant Staphylococcus bacteria (MRSA), and as a potential treatment for multidrug-resistant UTIs. Phage therapy can be highly personalized, as the concoction of phages can be tailored to work against the specific causative bacteria in each individual case. It may also be used in combination with antibiotics, further preventing the spread of resistance.
In order to widen the scope to develop alternative strategies against AMR even further, researchers also looked into the molecular mechanisms of AMR. The focus of this research is on the constant evolution and development of bacterial resistance against a battery of antibiotics, worryingly even the last-resort antibiotics such as carbapenem, vancomycin and colistin. To better understand the reasons behind this, researchers are studying the internal mechanisms and components of the bacteria, such as “jumping genes” or biofilms. Understanding these mechanisms will help them create more effective antibiotics against resistant bacteria.
Another exciting development in the research of antimicrobial resistance is the rise of Artificial Intelligence (AI), which can be used for AMR surveillance. Artificial intelligence is being used to maintain patient electronic health records as of 2024. This may allow authorities to detect patterns in the occurrence of resistant infections in patients, and therefore spot or predict outbreaks of resistant infections. It may also be used in analysing AMR-related data in further research.
Fighting resistance takes more than medicine
Increasing public awareness is also an important tool in the fight against antimicrobial resistance. It can be done for example in the form of education regarding the dangers of AMR and encouraging responsible antibiotic use. Antimicrobial resistance is also accelerated by poor access to water, sanitation, hygiene (WASH), and inadequate infection prevention and control in healthcare settings and communities. Promoting awareness among the public to practice cleanliness and hygiene can go a long way in keeping people infection-free, and hence antibiotic-free.
Due to the increasing instances of antibiotic-resistant infections, WHO updated the Bacterial Priority Pathogens List with a list of MDR bacteria. The bacteria are divided into critical, high, and medium priority pathogens based on the urgency to develop new antimicrobial strategies against them. The 2024 version is built upon the previous 2017 version, with added surveillance evidence and expert insights. The updated list allows us to focus on the resistant pathogens which need urgent attention, and against which new antimicrobial strategies need to be developed.
One of the determining factors in whether we win the battle against antimicrobial resistance is our attitude towards the world around us. The One Health approach recognizes the interconnectedness of human, animal, and environmental health. This holistic approach is also crucial in tackling the growing crisis of antimicrobial resistance. A One Health perspective highlights how antibiotic overuse in both humans and animals contribute significantly to the problem by ultimately increasing the load of antibiotics in the environment. The Food and Agriculture Organization of the United Nations (FAO), United Nations Environment Programme (UNEP), World Health Organization (WHO), and World Organisation for Animal Health (WOAH) are working collaboratively to address AMR through a One Health lens. They are also setting surveillance systems to track resistance patterns in bacterial infections, raising awareness of antimicrobial resistance and responsible antibiotic use in communities and healthcare facilities.
By promoting responsible antibiotic stewardship in all sectors, from human healthcare to livestock farming, we can slow down the development and spread of resistant microbes. We should reduce unnecessary antibiotic prescriptions in humans, implement better hygiene and biosecurity practices on farms to minimize disease outbreaks and the need for antibiotics, and manage antibiotic waste to prevent its release into the environment. The overuse of antibiotics in farm animals and fish to increase their weight should also be controlled to prevent indirect consumption of antibiotic residues via meat. Through collaborative, interdisciplinary efforts that sustain the health of humans, animals, and our environment, we can effectively manage antimicrobial resistance.
Researchers and health professionals battled against antimicrobial resistance with a multi-pronged approach in 2024. Antibiotics were approved for use by the Food and Drug Administration, and many more are in the pipeline for development. However, the battle against antimicrobial resistance is far from over. A lot has been done, but a lot more still needs to be done. We all play important roles in this global fight against resistant infections. The future of antibiotic efficacy, and indeed the future of global health, depends on the actions we take today. The journey is ongoing, but the progress made in 2024, combined with a concerted global effort, is a significant step in the right direction. Let’s seize this momentum and work together to ensure that antibiotics remain a powerful tool in our arsenal against infectious diseases for generations to come.
